Overview
Antimony (chemical symbol Sb) is a soft, lustrous, silver-grey metalloid. It is stable in air at room temperature, but reacts with oxygen when heated to form antimony trioxide (Sb2O3). It has a relatively low melting point of 630°C and a density of 6.697 g/cm3. Antimony is rare in the Earth's crust having a (upper) crustal abundance of only 0.4 ppm (Rudnick and Gao, 2003). Antimony is found in over 100 different mineral species, typically in association with elements such as mercury, silver and gold. The principal ore mineral of antimony is stibnite (Sb2S3).

| Global production | Global producers | EU consumption | EU share | EU suppliers | Import reliance |
|---|---|---|---|---|---|
| 106,878t | China 47% Tajikistan 20% Russia 16% Turkey 5% Myanmar 4% Bolivia 3% Australia 3% | 1,096t | 1% | Turkey 78% Bolivia 14% China 6% | 100% |
| Global production | Global producers | EU consumption | EU share | EU suppliers | Import reliance |
|---|---|---|---|---|---|
| 38,598t | China 46% Belgium 9% Vietnam 8% France 7% Tajikistan 5% Myanmar 4% Thailand 3% Korea, South 2% India 2% Bolivia 2% | 23,654t | 61% | Tajikistan 25% China 23% Belgium 12% Vietnam 12% France 8% Korea, South 5% Myanmar 3% Spain 3% Thailand 2% | 67% |
The evolution of the price of metallic antimony during the period 2000–2024 is presented in Figure 2. As shown, the price increased continuously and significantly, from €1,400 per tonne in 2000 to €19,300 per tonne in 2024. A sharp price peak is observed after 2009, the year of the global economic crisis. Since then, the price has remained at elevated levels. Another significant upward trend is noted in 2023.
Figure 3 presents the global supplier countries and the EU suppliers of antimony at both the extraction and processing stages. China is the major global supplier at both stages, accounting for 47% of global extraction and 45.8% of global processing. Tajikistan and Russia are significant suppliers at the extraction stage, while Belgium and Vietnam play important roles in the processing stage. Regarding EU suppliers, Turkey is the dominant source at the extraction stage, while Tajikistan, China, and Belgium are key suppliers at the processing stage.
The global end-of-life (EoL) recycling rate for antimony is estimated to be between 1 and 10% [UNEP,2013]. The Raw Materials Supply Assessment (RMSA) study, undertaken by BIO by Deloitte in 2015, suggests that the EoL recycling rate in the EU for antimony is as high as 28% [BIO by Deloitte,2015]. Secondary antimony is chiefly recovered from lead-acid batteries. Therefore, the availability of secondary antimony is almost entirely dependent on the extent of lead recycling and the market conditions for lead and lead-acid battery scrap.
Around 43% of antimony (in the form of antimony trioxide, or ATO) is used in flame retardancy. Antimonial (or hard-lead alloys) are used in the manufacture of lead-acid batteries, accounting for about 32% of global antimony consumption. Around 6% of antimony, in the form of antimony trioxide (ATO), is used as a catalyst in the production of polyethylene terephthalate (PET). Antimony in the form of sodium hexahydroxyantimonate, is used in the manufacture of high-quality clear glass. This use accounts for about 5% of the global antinomy consumption.
| Use | Percentage | Substitute | Sub share | Cost | Performance |
|---|---|---|---|---|---|
| Flame retardants | 43% | No substitute | 70% | No substitute | |
| Flame retardants | 43% | Boron oxide | 10% | Similar or lower costs | Reduced |
| Flame retardants | 43% | Zinc oxide | 10% | Similar or lower costs | Reduced |
| Flame retardants | 43% | Hydrated aluminium oxide | 10% | Similar or lower costs | Reduced |
| Lead-acid batteries | 32% | No substitute | 50% | No substitute | |
| Lead-acid batteries | 32% | Copper | 10% | Similar or lower costs | Similar |
| Lead-acid batteries | 32% | Calcium | 10% | Similar or lower costs | Similar |
| Lead-acid batteries | 32% | Tin | 10% | Very high costs (more than 2 times) | Similar |
| Lead-acid batteries | 32% | Sulfur | 10% | Similar or lower costs | Similar |
| Lead-acid batteries | 32% | Selenium | 10% | Very high costs (more than 2 times) | Similar |
| Lead alloys | 14% | No substitute | 50% | No substitute | |
| Lead alloys | 14% | Selenium | 10% | Very high costs (more than 2 times) | Similar |
| Lead alloys | 14% | Copper | 10% | Similar or lower costs | Similar |
| Lead alloys | 14% | Calcium | 10% | Similar or lower costs | Similar |
| Lead alloys | 14% | Tin | 10% | Very high costs (more than 2 times) | Similar |
| Lead alloys | 14% | Sulfur | 10% | Similar or lower costs | Similar |
| Plastics (catalysts and stabilisers) | 6% | not assessed under 10% | 100% | No substitute | |
| Glass and ceramics | 5% | not assessed under 10% | 100% | No substitute |
Table 3 presents various components and metals that could potentially be used as substitutes for antimony oxide and metallic antimony. Zinc, aluminum, and boron oxides can be used as flame retardant substitutes for antimony oxide; however, their use is limited to a maximum of 10% of applications.
Several elements—such as copper, calcium, tin, sulfur, and selenium—can partially replace antimony in lead-acid batteries, although this substitution is also limited in scope. Similarly, in lead alloys, antimony can be substituted in certain applications by copper, calcium, tin, and sulfur, but only to a limited extent.
Finally, no viable substitutes for antimony have been identified in plastics, glass, or ceramics.
lead-acid battery manufacturers have initiated research and development programs that could ultimately lead to significant decreasing of the use of both lead and antimony in lead-acid battery alloys. Consumption of antimony for batteries in North America has declined over the past few decades as many battery designsare manufactured with alloys of lead with calcium, selenium, or tin instead of antimony owing to performance and price advantages [USGS ANTIMONY,2025].
On the contraty, Sb demand has risen due to increasing industrial use and China’s dominance in production. Antimony is crucial in perovskite-based solar panels. China is the biggest antimony producer, but its output has dropped sharply in 2023 mainly due to mine closures and stricter environmental rules. Russia is also a major Sb producer. After Russia’s 2022 invasion of Ukraine have made trading with Russian suppliers more difficult, further tightening global supply. Global demand for antimony is projected to grow from $2.5 billion in 2024 to $3.5 billion by 2030, at a CAGR of 6.2%.U.S. antimony market is expected to expand significantly, reaching an estimated value of USD 106.57 million by 2032. This growth primarily be driven by the rising demand for OSHA-regulated flame-retardant clothing. Concerning the antimony supply:Australia is expected to increse the production Australia. Larvotto Resources, which runs the Hillgrove Gold-Antimony Project will be a major antimony producer player. Spearmint Resources in Canada has doubled its acreage at the George Lake South Antimony Project, recognizing the mineral’s strategic value. Furthemore, Tajikistan is an emerging producer. Dzheitun and Konchok in Tajikistan, owned by a U.S. company, is now Europe’s largest supplier of the metal [carboncredits,2025].
Antimony (Sb) is a potentially toxic metalloid and is released into the environment through various pathways, including mining, ore transportation, smelting, manufacturing, and use of their products, disposal of wastes, and sludges, wastewater, and so on [Chu et al. ,2019] [Stan?i? et al. ,2022]. Studies indicate that antimony is retained in the soil through adsorption and can sorb onto clay minerals, oxides, and hydroxides in the soil and aquatic sediment (ATSDR 2019). Strong enrichment of soils in Sb can pose a considerable risk to the environment. It should be stressed, however, that real hazards will depend on Sb solubility rather than on its total concentrations [Lewinska et al.,2018]. Workplace exposure limit values are in place for one or more forms of antimony. In Europe, the most accepted limit is 0.5 mg/m³, but more severe limits exist, such as the 0.25 mg/m³ in Sweden [GESTIS,NA]. However, some agencies are revising the existing limits and calculating new ones that involve respirable occupational exposure limits (OEL) instead of inhalable OELs. In 2018 the German BAuA (Federal Institute for Occupational Safety and Health) published a limit of 0.006 mg respirable Antimony/m³ for Antimony trioxide and Antimony trisulfide as part of the German TRGS 900 (Technical Rules for Hazardous Substances) [International Antimony Association,NA].
Market analysis, trade and prices
| Global production | Global producers | EU consumption | EU share | EU suppliers | Import reliance |
|---|---|---|---|---|---|
| 106,878t | China 47% Tajikistan 20% Russia 16% Turkey 5% Myanmar 4% Bolivia 3% Australia 3% | 1,096t | 1% | Turkey 78% Bolivia 14% China 6% | 100% |
| Global production | Global producers | EU consumption | EU share | EU suppliers | Import reliance |
|---|---|---|---|---|---|
| 38,598t | China 46% Belgium 9% Vietnam 8% France 7% Tajikistan 5% Myanmar 4% Thailand 3% Korea, South 2% India 2% Bolivia 2% | 23,654t | 61% | Tajikistan 25% China 23% Belgium 12% Vietnam 12% France 8% Korea, South 5% Myanmar 3% Spain 3% Thailand 2% | 67% |
Antimony is traded in several forms such as ores and concentrates, antimony trioxide (ATO), unwrought antimony metal and powders, and scrap. As halogenated antimony trioxide is still highly used as an effective flame retardant, ATO is likely to remain the principal market for antimony in the EU. The continued use of ATO in flame retardants is also likely to be driven by increasingly stringent fire regulations. The use of antimony in lead-acid batteries is estimated to decrease as various producers substituted antimony in this application on environmental grounds in many developing nations [crmalliance.eu,NA] [van den Brink et al.,2022]. Global consumption of antimony is expected to increase from 2016 to 2020, primarily in the use applications: flame retardants, lead-acid batteries, and plastics. Asia is projected to remain the leading region regarding consumption, accounting for about 60% of global consumption by 2021 [USGS ANTIMONY,2025]. However, antimony production (both extraction and processing) was reduced in 2021 due to environmental audits in China, and various temporary mine shutdowns during the global COVID-19 pandemic [USGS ANTIMONY,2025]. A new antimony plant in Oman was planned to operate in 2019. The plant was set up to treat 40,000 tonnes per year of antimony-gold concentrates producing 20,000 tonnes per year of antimony metal and antimony trioxide, making it the largest antimony roaster outside of China. Nevertheless, according to experts, most of the conversion of antimony ores into antimony metal or ATO would occur in China [van den Brink et al.,2022].
| Mining | Processing/refining | ||
|---|---|---|---|
| CN Code | Title | CN Code | Title |
| 26171000 | Antimony ores and concentrates | 28258000 | Antimony oxides |
| 81101000 | Unwrought antimony; antimony powders | ||
The CN codes 26171000, 28258000 and 81101000 are identified corresponding to antimony ores and concentrates, antimony oxides and unwrought Sb/Sb powders, respectively. CN 28258000 (antimony trioxide) consists an end-product used as flame retardant in combination to halogenated materials.
Figure 5 illustrates the quantities of unwrought antimony and antimony powders imported to and exported from the EU between 2002 and 2024. The following observations can be made:
(a) Imports ranged from approximately 16,000 to 25,000 tonnes;
(b) Exports were negligible in comparison, not exceeding around 500 tonnes.
Figure shows the evolution of EU imports of unwrought antimony and antimony powders over the same period. Total import volumes fluctuated between 20,000 and 25,000 tonnes. China was historically the EU’s primary import partner until 2019. Since then, the EU has diversified its sources, importing from a broader range of countries.
In 2024, Tajikistan emerged as the leading supplier, accounting for 12,500 tonnes. Vietnam and Thailand also became significant sources, with import volumes of 2,100 tonnes and approximately 1,500 tonnes, respectively.
Figure 7 shows the imported and exported quantities of antimony oxides in the EU during the period 2000–2024. The following observations can be made:
-
From 2000 to 2009, imports (reaching up to 9,300 tonnes) exceeded exports (which reached up to 5,600 tonnes).
-
Since 2009, exports have surpassed imports. According to the most recent data (2024), exports are estimated at approximately 7,400 tonnes, while imports are around 3,700 tonnes.
Figure 8 presents the EU’s import partners of antimony oxides during the same period. China has been the major import partner throughout the entire timeframe. However, imports from China have significantly declined after 2007 — from about 7,700 tonnes in 2007 to approximately 2,100 tonnes in 2024. Additionally, a notable quantity (around 1,400 tonnes) is imported from various unspecified countries.
Figure 9 shows the imported and exported quantities of antimony ore and concentrates in the EU during the period 2000–2024. The following observations can be made:
-
Throughout the entire period, import volumes consistently exceeded export volumes, with significant fluctuations observed in the imported quantities.
-
Imports dropped significantly from approximately 2,400 tonnes in 2000 to about 800 tonnes in 2002. A gradual increase occurred after 2011, peaking at around 1,700 tonnes in 2014.
-
Export volumes remained very limited, reaching a peak in 2012 at approximately 1,100 tonnes. Since 2019, exports have dropped to just a few dozen tonnes per year.
Figure 10 indicates that Turkey and Bolivia were the most significant import partners during the examined period. Imports from Bolivia gradually declined after 2019 and ceased entirely after 2022.
The evolution of elemental antimony prices during the period 2000–2024 is shown in Figure 11. As can be observed, prices increased progressively throughout the entire period. Notably, there was a sharp spike in 2009 — the year of the global economic crisis — from approximately €3,700 per tonne to €10,600 per tonne. Prices remained at elevated levels between 2013 and 2023, ranging from €6,000 to €12,700 per tonne. A dramatic further increase was observed after 2023, reaching approximately €19,400 per tonne in 2024. This trend is largely attributed to Chinese export restrictions.
OUTLOOK FOR SUPPLY
China is main supplier of the antimony and will retain this position in next decade due to the availability of antimony refining and processing infrastructure. However, China antimony producers nowadays heavily rely on imported antimony concentrates (with Russia and Tajikistan as the main sources) since some of the main Chinese antimony mines have been depleted [Perpetua Resources,2021] [Fastmarkets.com,2022]. Chinese antimony production from 2010 to 2020 decreased indeed for more than 50 % - from 130,000 t in 2010 to 61.000 t in 2020 [WMD,2022]. Main reason for that is, alongside depletion of domestic antimony mines, also the China Green Shield Strategy and ecological inspections in the mines and refineries, which restricts antimony processing and mine supply if the ecological standards are not met [markets.financialcontent.com,2025]. This urged the Chinese authorities to strategically reduce output at local antimony mines to avoid additional depletion of domestic supplies. Chinese investment companies also secured controlling interests in antimony projects in South Africa (Murchison Belt), Bolivia, Australia, Tajikistan and Canada [Perpetua Resources,2021]. A major antimony roasting plant outside of China is the SPMP (Strategic & Precious Metals Processing) project in Oman. It has the annual capacity of 20,000 t of antimony products (metal and trioxide) [SPMP,NA]. Russian invasion on Ukraine will have additional impact on antimony market on the supply side as well as on the demand side stockpiling of antimony as national strategic reserves and increased consumption in military sector [Kadam, T.,2022]. Antimony market size is expect to exceed 105 ktonnes in 2030 corresponding to a 1.5% CAGR during the period 2025-2030. The production will be mainly driven by China (estimated share between 41 and 47%) [RFC Ambrian,2025] [mordorintelligence.com (antimony),2025].
Demand
According to most of forecasts, antimony market size is expect to exceed 105 ktonnes in 2030 corresponding to a 1.5% CAGR during the period 2025-2030 [RFC Ambrian,2025], [mordorintelligence.com (antimony),2025]. However, some other estimations are less optimistic estimating that global demand for antimony is estimated at USD 2.5 billion in 2024 and is projected to reach USD 3.5 billion by 2030, growing at a CAGR of 6.2% over the forecast period [globenewswire.com,2024].
The demand for antimony is primarily driven by its extensive use in flame retardants, lead-acid batteries, electrical and electronic components, and plastic additives. The form in which antimony is used—whether as trioxide, pentoxide, or alloy—depends on the application [globenewswire.com,2024].
For instance, antimony trioxide and pentoxide are key components in flame retardants, which are widely used in the production of construction materials, plastics, textiles, and electronic components.
In the energy sector, antimony-lead alloys are commonly used in lead-acid batteries to improve strength, corrosion resistance, and castability of the battery grids. These batteries are extensively deployed in:
Vehicles (primarily for starting, lighting, and ignition)
Renewable energy storage systems
Uninterruptible power supplies (UPS)
Telecommunications
Off-grid housing
While lithium-ion batteries dominate the market for electric vehicles (EVs), lead-acid batteries continue to play an important role—especially for engine starting, standby power, and auxiliary loads.
The antimony ore market in the EU is depicted in Figure 12, which presents domestic production, demand, imports, and exports during the period 2000–2024. As can be observed, there has been no primary production throughout the entire period studied. Demand has increased from approximately 570 tonnes in 2003 to about 1,400 tonnes in 2024 and has been fully met through imports.
Figure 13 illustrates the EU market for antimony oxides and raw elemental antimony, showing domestic production, demand, imports, and exports from 2000 to 2024. Demand rose sharply after 2001, increasing from approximately 5 kilotonnes (kt) in 2001 to 31.5 kt in 2002. Significant domestic production—reaching up to around 20 kt—occurred during the period 2016–2020, primarily at the processing stage. Outside of this period, demand has been almost entirely met through imports, which have ranged between 29.5 kt and 33 kt.
Approximately 43% of antimony (primarily in the form of antimony trioxide, or ATO) is used in flame retardants. Antimonial alloys (also known as hard-lead alloys) are used in the manufacture of lead-acid batteries, accounting for about 32% of global antimony consumption. Around 6% of antimony, also in the form of ATO, is used as a catalyst in the production of polyethylene terephthalate (PET). Additionally, antimony in the form of sodium hexahydroxyantimonate is used in the production of high-quality clear glass, representing about 5% of global antimony consumption.
Figures 14 and 15 present antimony consumption by application at the global and European levels, respectively. As shown, the use of antimony trioxide (Sb?O?) as a flame retardant constitutes the primary application both worldwide and within the EU. The second most significant use is metallic antimony as an alloying element in lead-antimony (Pb-Sb) alloys for lead-acid batteries.
Antimony consumption for Pb-Sb alloys is higher in the EU than the global average, likely due to the large capacity of the European battery manufacturing sector. Other notable applications include its use as a catalyst and stabilizer in plastics, as a component in non–Pb-Sb alloys, and in glass production.
| Applications | 2-digit NACE sector | Value added of NACE 2 sector | 4-digit CPA |
|---|---|---|---|
| Flame retardants | C20 - Manufacture of chemicals and chemical products | 165,880M€ | C20 - Manufacture of chemicals and chemical products |
| Lead-acid batteries | C27 - Manufacture of electrical equipment | 100,100M€ | C27 - Manufacture of electrical equipment |
| Lead alloys | C25 - Manufacture of fabricated metal products, except machinery and equipment | 216,840M€ | C25 - Manufacture of fabricated metal products, except machinery and equipment |
| Plastics (catalysts and stabilisers) | C20 - Manufacture of chemicals and chemical products | 165,880M€ | C20 - Manufacture of chemicals and chemical products |
| Glass and ceramics | C23 - Manufacture of other non-metallic mineral products | 86,399M€ | C23 - Manufacture of other non-metallic mineral products |
The gross added value per antimony-related sector in the EU during the period 2011–2022 is presented in Figure 16. The manufacture of metallic products containing antimony (e.g., lead–antimony alloys) is the sector with the highest added value, reaching €200 million in 2024. This is followed by the manufacture of chemical products, electrical equipment, and non-metallic mineral products, with added values of approximately €165.8 million, €100 million, and €86.4 million, respectively.
-
Around 43% of antimony (in the form of antimony trioxide, or ATO) is used in flame retardancy.
Antimony-based flame retardants are used in plastics, cable coatings, upholstered furniture, car seats, fabrics and household appliances [Gann and Gilman,2003].
Antimony trioxide, which is not is not a flame retardant in itself, is used as a co-synergist with halogenated (i.e., brominated or chlorinated) flame retardants to enhance their effectiveness as retardant compounds, achieved by hindering the chain reaction of the flame gas phase through stepwise release of the halogenated radicals, which inhibit ignition and pyrolysis in solids, liquids and gases.
They also promote the formation of a char-rich layer on the substrate, which reduces oxygen availability and volatile-gas formation [Schwarz-Schampera,2014].
Antimonial (or hard-lead alloys) are used in the manufacture of lead-acid batteries, accounting for about 32% of global antimony consumption,
The incorporation of 1-15 % antimony in these alloys improves tensile strength and thus charging characteristics. Further, it also prevents the production of unwanted hydrogen during charging.
Antimony-lead alloys that contain 1-3% antimony are easy to cast and are used in the production of grid plates, straps and terminals in lead-acid batteries [Schwarz-Schampera,2014].
The production of lead alloys accounts for about 14% of global antimony use.
These alloys are used in the manufacture of low-load bearings applications in the automotive sector and io household and decorative items (such as teapots, vases and lamp stands.)
Tin-lead-antimony solders are used extensively in the electronics industry [Schwarz-Schampera,2014].
-
Around 6% of antimony, in the form of antimony trioxide (ATO), is used as a catalyst in the production of polyethylene terephthalate (PET).
PET is one of the key input materials for the manufacture of plastic bottles, also for water and food bottles.
ATO is also used as a heat stabilizer in polyvinyl chloride (PVC) [Schwarz-Schampera,2014].
Antimony in the form of sodium hexahydroxyantimonate, is used in the manufacture of high-quality clear glass. This use accounts for about 5% of the global antinomy consumption. In this particular application, antimonates are primarily used as degassing agents, which act to remove trapped air bubbles from the cooling glass. They also act as a fining agent for removing impurities (e.g. iron) that may produce unwanted colouration [Schwarz-Schampera,2014].
| Use | Percentage | Substitute | Sub share | Cost | Performance |
|---|---|---|---|---|---|
| Flame retardants | 43% | No substitute | 70% | No substitute | |
| Flame retardants | 43% | Boron oxide | 10% | Similar or lower costs | Reduced |
| Flame retardants | 43% | Zinc oxide | 10% | Similar or lower costs | Reduced |
| Flame retardants | 43% | Hydrated aluminium oxide | 10% | Similar or lower costs | Reduced |
| Lead-acid batteries | 32% | No substitute | 50% | No substitute | |
| Lead-acid batteries | 32% | Copper | 10% | Similar or lower costs | Similar |
| Lead-acid batteries | 32% | Calcium | 10% | Similar or lower costs | Similar |
| Lead-acid batteries | 32% | Tin | 10% | Very high costs (more than 2 times) | Similar |
| Lead-acid batteries | 32% | Sulfur | 10% | Similar or lower costs | Similar |
| Lead-acid batteries | 32% | Selenium | 10% | Very high costs (more than 2 times) | Similar |
| Lead alloys | 14% | No substitute | 50% | No substitute | |
| Lead alloys | 14% | Selenium | 10% | Very high costs (more than 2 times) | Similar |
| Lead alloys | 14% | Copper | 10% | Similar or lower costs | Similar |
| Lead alloys | 14% | Calcium | 10% | Similar or lower costs | Similar |
| Lead alloys | 14% | Tin | 10% | Very high costs (more than 2 times) | Similar |
| Lead alloys | 14% | Sulfur | 10% | Similar or lower costs | Similar |
| Plastics (catalysts and stabilisers) | 6% | not assessed under 10% | 100% | No substitute | |
| Glass and ceramics | 5% | not assessed under 10% | 100% | No substitute |
Table 8 presents various components and metals that could potentially be used as substitutes for antimony oxide and metallic antimony. Zinc, aluminum, and boron oxides can be used as flame retardant substitutes for antimony oxide; however, their use is limited to a maximum of 10% of applications.
Several elements—such as copper, calcium, tin, sulfur, and selenium—can partially replace antimony in lead-acid batteries, although this substitution is also limited in scope. Similarly, in lead alloys, antimony can be substituted in certain applications by copper, calcium, tin, and sulfur, but only to a limited extent.
Finally, no viable substitutes for antimony have been identified in plastics, glass, or ceramics.
-
Major substitutes of halogenated flame retardants (and antimony trioxide) are mineral fillers, both aluminium and magnesium hydroxides.
These fillers yield crystallization water at higher temperatures, achieving a certain flame retardancy, but at high levels of filling in the range of 150–200 parts of the hydroxide per 100 parts of, for example, unsaturated polyester resin, it is possible to achieve self-extinguishing and a low smoke density. The disadvantage of such systems is that the entire material has a high density and whilst applications such as plastics, cable sheathing and carpets can tolerate some level of mineral filler, fabrics cannot.
As a replacement for antimony in halogenated fire-retardant systems there is the potential use of zinc hydroxystannate or zinc borate at similar levels, however they are only effective in certain systems and not as good as antimony trioxide [Gann and Gilman,2003].
Pb-Ca-Sn alloys containing 0.08 wt.% calcium and up to1.5% Sn have been proposed as substitutes of the lead-antimony alloy in lead-acid batteries [Kamenev et al. ,2002].
Several metals can substitute for antimony in the production of lead alloys – including cadmium, calcium, selenium, tin and copper.
The properties of a given alloy are not controlled by a single metal, but rather by the combination of several metals, where each metal may produce a range of effects in the alloy [Schwarz-Schampera and Herzig,2002] [crmalliance.eu,NA]. Accordingly, any substitution would be associated with a price and/or performance penalty which, in general, means that there appears to be little economic or technical incentive to substitute antimony in its principal applications.
Various combinations of cadmium, barium, calcium, lead, tin, zinc and germanium may substitute for antimony in the production of plastics, where antimony acts as a stabiliser or catalyst, but this option is commonly more expensive.
Compounds of chromium, tin, titanium, zinc and zirconium can substitute for antimony in the manufacture of pigments and glass.
Cadmium, chromium, tin, titanium, zince and zirconium have been described as antimony substitutes in the manufacture of ceramics and glass pigments. These substitutes have not been commercialized so far [ BLAZY and HERMANT,2015].
Supply
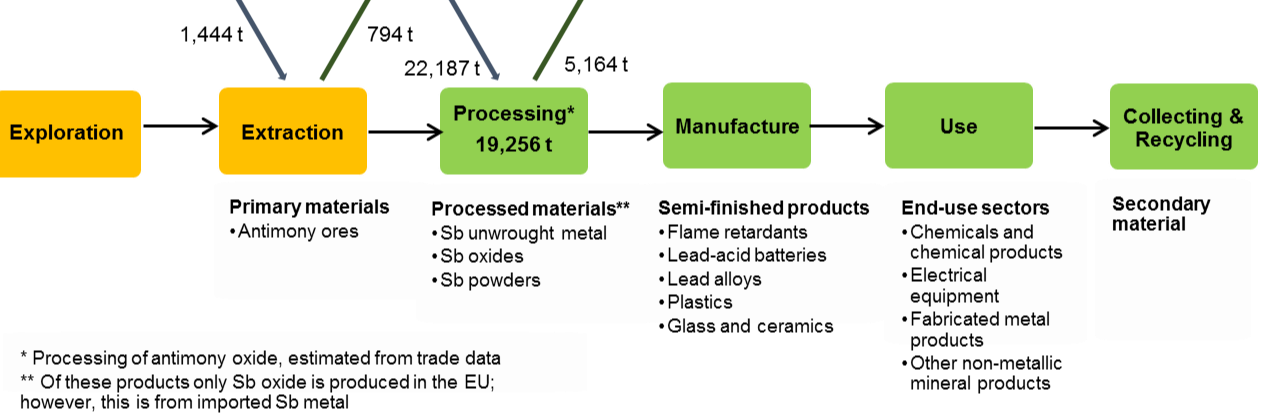
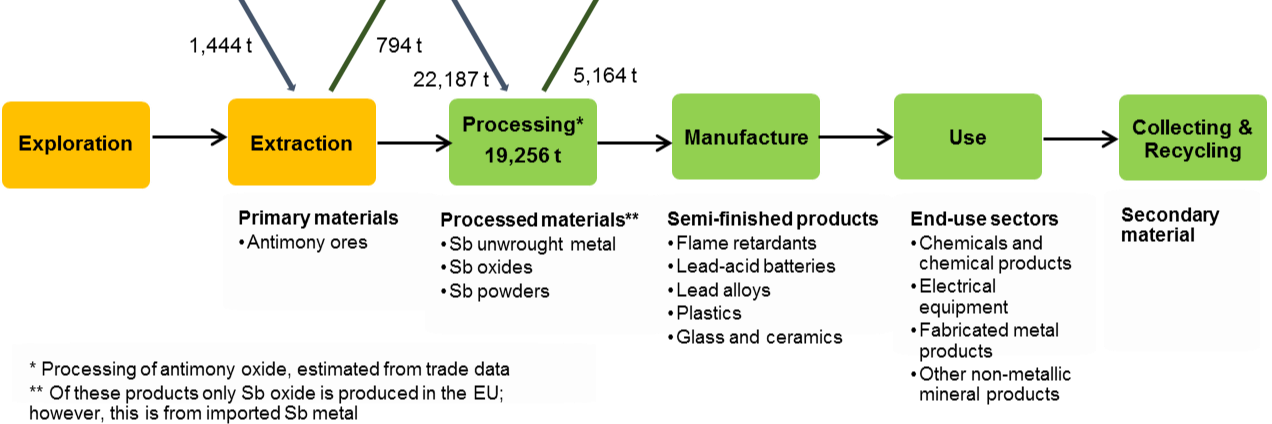
The flows of antimony through the EU economy in 2012 are demonstrated in Figure 10 [BIO Intelligence Service,2015].
Antimony forms a wide range of minerals from native Sb (100 wt.% Sb), most common ore mineral stibnite (72 wt.% Sb) as well as antimony oxides valentinite (83 wt.% Sb) and its polymorph senarmontite (88 wt.% Sb) to the vide array of sulfosalts such as chalcostibite (49 wt.% Sb), famatinite (27 wt.% Sb), pyrargyrite (23 wt.% Sb), tetrahedrite (30 wt.% Sb), jamesonite (35 wt.% Sb), boulangerite (26 wt.% Sb), bournonite (25 wt.% Sb) etc. [Dill, H.G.,2010].
The most important types of the antimony deposits, based on their ore resources, include: (1) greenstone-hosted quartz-carbonate veins and carbonate replacement deposits; (2) gold-antimony (epithermal) deposits; and (3) reduced magmatic gold systems. In many of these deposits, stibnite (Sb2S3) is the principal ore mineral.Greenstone-hosted antimony deposits are of particular economically importance. They are estimated to tens of millions of tonnes in size and typically contain between 1.5 and 25% Sb2S3. These deposits typically comprise a stockwork of gold-antimony-quartz-carbonate veins hosted by metavolcanic and/or metasedimentary rocks. Carbonate replacement deposits are also found in some of these metasedimentary sequences (e.g. Xikuangshan, China), which are thought to form by hydrothermal alteration of the host material. Epithermal gold-antimony deposits are generally smaller than greenstone-hosted deposits. They are typically up to 1 million tonnes in size and have lower ore grades (up to 3.5% Sb2S3). The formation of these epithermal deposits is linked to the emplacement of magmatic porphyry copper systems in the shallow crust (upper 1.5 km). The mineralisation generally takes the form of veins, or disseminations of stibnite and/or tetrahedrite ((Cu,Fe)12Sb4S13) in the host rocks. Reduced magmatic gold systems are associated with the intrusion of metaluminous granite plutons, the mineralisation taking the form of quartz-carbonate sheeted veins, replacement bodies and/or skarns. The mineralisation may be enriched in several metals, including gold, tellurium, tungsten, arsenic and antimony. These deposits are similar in size to the greenstone-hosted antimony deposits, but have typically much lower grades (0.1 to 1.5% Sb2S3) [Schwarz-Schampera,2014].
| Country | Reserves (tonnes) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The global antimony reserves by country are displayed in Figure 9 [USGS ANTIMONY,2025].
xxxxxxxxxxxxxxxx
|
According to the data collected in the frame of the E-mineral yearbook in Mintell4EU project published on EGDI portal, only two countries reported antimony resources in 2019 – France and Sweden (EGDI, 2022). In the previous data reporting in 2013, five countries - France, Sweden, Finland, Slovakia and Greece reported antimony resources.
Most resource figures in Europe are based on historic estimates and thus not reported in accordance with the UNFC system of reporting. These resources are currently considered to be of little economic interest. Germany also holds antimony resources, but data are not reported because data collection in that country is under the responsibility of authorities at federal state level (Minerals4EU, 2019). Resource data for some countries in Europe are available on the EGDI portal (see EGDI, 2022) but cannot be summed as they are partial, and they do not use the same reporting code [Minerals4EU,2019].
Other than the resource estimation reported on Minerals4EU website, antimony resources are reported in Rockliden, Sweden with 0,8 Mt of indicated mineral resources with 0.18% Sb (also contains 4.04% Zn, 2.1%).
| Country | Classification | Quantity (Mt of ore) | Grade (% Antimony) | Reporting code | Reporting date | Source | ||
|---|---|---|---|---|---|---|---|---|
| aaaaaaaaaaaa |
New Antimony Projects in the EU
The exploitation of antimony deposits within the European Union has recently attracted increased attention, particularly due to the metal's critical role in various military and strategic applications. These include flame-retardant fabrics, communication equipment, night vision goggles, ammunition, and laser sighting systems [investingnews antimony,NA].
The most significant antimony-related projects currently underway in the EU are as follows:
a) The AUREOLE Project
Led by BRGM (the French Geological Survey) in collaboration with institutions from Spain and Portugal, the AUREOLE project aims to develop a 3D metallogenic model for antimony (Sb) mineralization in the Paleozoic terrane of the Iberian Peninsula [aureole,2022].
b) Slovakia – Pezinok and Tiennesgrund Deposits
There is renewed interest in the re-evaluation and potential exploitation of the historical antimony mine in Pezinok, located in western Slovakia. This initiative is being considered alongside a second occurrence in Tiennesgrund, also in Slovakia [Writer. S.,2024].
c) Greece – Kermatoti Mine (Chios Island)
The Greek government has issued a call for expressions of interest for the exploitation of the historical Kermatoti antimony deposit on Chios Island [Kokkinidis,2025].
These initiatives are still in the early exploration or evaluation stages. However, if successfully developed, they could enable antimony production within the EU in the medium term, contributing to strategic autonomy in critical raw materials.
According to the WMD [World Mining Data,2025], the global mine production of antimony increased to approximately 105?kt in 2024, following a period of continuous decline since 2017. (Figure 18). China is the largest supplier of antimony ores and concentrates, accounting for approximately 42% of global production in 2024. However, its output has declined significantly—from a peak of 163?kt in 2007 to just 41?kt in 2024. On the contrey Tjikistan and Russia have become major antimony producers since 2018. Antimony production in Russia especially incresed from less than 5 kt in 2022 to >32 kt in 2024 possibly due to various applications of antimony in the millitary sector.
Slightly different data regarding the global trend in primary antimony production are reported by the USGS [USGS ANTIMONY,2025] (Figure 19). According to this source, global antimony production appears to have stabilized at approximately 115?kt during the period 2020–2022.
| MSA Flow | Value | ||
|---|---|---|---|
The global end-of-life (EoL) recycling rate for antimony is estimated to be between 1 and 10% . The Raw Materials Supply Assessment (RMSA) study, undertaken by BIO by Deloitte in 2015, suggests that the EoL recycling rate in the EU for antimony is as high as 28% [BIO Intelligence Service,2015]. Secondary antimony is chiefly recovered from lead-acid batteries. Therefore, the availability of secondary antimony is almost entirely dependent on the extent of lead recycling and the market conditions for lead and lead-acid battery scrap. Since the supply of primary antimony is heavily concentrated in a few countries, the recovery of secondary antimony is an important part of the supply chain in countries like, for example, the United States, Japan, Canada and the EU. On a global scale, it was estimated, that in 2010 the secondary production of antimony accounted for about 20% of total antimony supply [Sundqvist Ökvist, L. ,2018]. In the EU, there are companies dealing with secondary antimony. Umicore is a company headquartered in Belgium, which recovers antimony from end-of-life batteries, mostly from electric cars. At Umicore antimony is recovered from complex lead-bearing concentrates as well as various complex residues from the lead/copper/zinc industry. The antimony is extracted during the lead refining process in the form of sodium antimonite [Umicore, Antimony,NA]. Solvay in France recycles halophosphate from spent fluorescent batteries [Sundqvist Öqvist, P.?L., et al.,2018] . Antimony used in the manufacture of plastics and flame retardants is generally not recovered because antimony is dispersed in these products [Schwarz-Schampera,2014]. However, antimony could potentially be recovered from the bottom ash resulting from the incineration of some of these products at their end-of-ife stage, but this currently does not appear to be economically viable [Daigle and DeCarlo,2021]. Asia Pacific is projected to dominate the global recycled antimony market during the forecast period. This can be ascribed to the increase in demand for Sb-containing packing alloys in several industries such as food & beverages. Strong economic growth, rise in standard of living, and increase in demand for packaging food are key factors propelling the demand for packaging materials in food & beverages industries. Furthermore, increase in usage of antimony flame retardants, lead-acid batteries, and plastics is estimated to drive the antimony market in Asia Pacific in the near future [RFC Ambrian,2025].
Antimony could potentially be recovered from the bottom ash resulting from the incineration of some of these products at their end-of-ife stage, but this currently does not appear to be economically viable [Dupont et al. ,2016].
Antimony ore is initially beneficiated-enriched via various methodologies. Hand selection, gravity separation and flotation are the most commonly applied techniques. Gravity separation is one of the most economical and effective antimony beneficiation methods. It can also be used as a pre-enrichment operation for flotation, reducing the burden on grinding equipment and improving the efficiency of the process flow. The embedded particle size of antimony ore is generally coarse, and the specific gravity of antimony crystal is much larger than that of the associated gangue. Flotation is also an efficient technique since stibnite is an easily floated mineral. Butyl xanthate or a mixture of shale oil and ethyl thiazide, and the foaming agent is pine oil or No. 2 oil are used as flotation reagents [mineraldressing.com,2022].The second step comprises the oxidative roasting of the stibnite concentrates for the conversion of Sb sulphide (Sb2S3) to Sb oxide (Sb2O3) in rotary kilns at 1100-1200oC. Alternatively, the removal of sulphur can be performed via flash volatilization oxidative roasting of via volatilization smelting in blast furnace. In last case stibnite is smelted. In order to produce metallic Sb, the oxidized concentrate is submitted to reductive smelting in blast furnace. A more complex methodology is followed in case of Au-rich stibnite ores for the extraction of both Sb and Au. The antimony-gold ore, lime, and coke were smelted in the blast furnace where more than 90% of antimony was volatilized to crude antimony oxide and about 10% antimony metal stayed in the BF, as a gold carrier. Subsequently, the crude antimony was oxidized to produce enriched gold-antimony alloy which was further treated via chlorination leaching. The resulted antimony oxide is also processed by reduction smelting and oxidation blowing to produce antimony oxide and gold-antimony alloy containing high concentrations of lead. The antimony oxide was processed in the reverberatory furnace to produce antimony metal (Figure 19) [Moosavi-Khoonsari et al.,2022].
Figurexxx. Flowsheet for the processing of Au-rich stibnite for the extraction of metallic antimony and gold [Moosavi-Khoonsari et al.,2022].
ESG DATA
Based on production volume, almost 70% of antimony was mined as by- or co-product in 2018, indicating a high supply risk. Antimony is a heavy metal that can be possibly has a negative impact of human health and can cause significant environmental pollution, both by mining and disposal. The disposal of PV panels will become a salient environmental issue in the next decades. The need for resilience and particularly substitution should therefore be considered not only from an economic point of view, but also from an environmental and health perspective [van der Brink,2022].
Other considerations
Workplace exposure limit values are in place for one or more forms of antimony. In Europe, the most accepted limit is 0.5 mg/m, but more severe limits exist, such as the 0.25 mg/m in Sweden [GESTIS,NA]. However, some agencies are revising the existing limits and calculating new ones that involve respirable occupational exposure limits (OEL) instead of inhalable OELs. In 2018 the German BAuA (Federal Institute for Occupational Safety and Health) published a limit of 0.006 mg respirable Antimony/m3 for Antimony trioxide and Antimony trisulfide as part of the German TRGS 900 (Technical Rules for Hazardous Substances) [International Antimony Association,NA]. Directive 2009/48 relating to the safety of toys establishes three migration limits for Antimony in toys: 45 mg/kg in dry, brittle, powder-like or pliable toy material, 3 mg/kg in liquid or sticky toy material and 560 mg/kg in scraped-off toy material. Due to its toxicity for reproduction antimony is listed as a Substance of Very High Concern under REACH in annex XIV [ECHA, antimony,NA].
Antimony (Sb) is a potentially toxic metalloid and is released into the environment through various pathways, including mining, ore transportation, smelting, manufacturing, and use of their products, disposal of wastes, and sludges, wastewater, and so on [Stan?i? et al. ,2022]. Studies indicate that antimony is retained in the soil through adsorption and can sorb onto clay minerals, oxides, and hydroxides in the soil and aquatic sediment [ATSDR, antimony,2019]. Strong enrichment of soils in Sb can pose a considerable risk to the environment. It should be stressed, however, that real hazards will depend on Sb solubility rather than on its total concentrations [Lewinska et al.,2018]. The following waste and environmental restrictions are established in the EU [International Antimony Association,NA]. The decision 2000/532/EC of 3 May 2000 stipulates that Antimony Trioxide shall be classified as "heavy metal" in the classification of hazardous waste. The decision 2003/33/EC establishing criteria and procedures for the acceptance of waste at landfills, gives leaching limit values for antimony from waste acceptable at landfills for inert waste. The Directive 2000/76/EC on the incineration of waste, and the EU Directive 2010/75 on Industrial Emissions and its best available techniques associated emissions limit values (BAT-AELs), include maximum air emission limit values for Antimony as well as for other possible emissions.
In 2015, the government of the Popular Republic of China issued the Emission standards of pollutants for stannum, antimony and mercury industries. The standard was formulated in a bid to implement laws and regulations including the Environmental Protection Law, the Law on the Prevention and Control of Water Pollution, the Law on the Control of Atmospheric Pollution and Marine Environment Protection as well as the Air Pollution Prevention and Control Action Plan. The standard stipulates the ceiling value for the discharge of water and air pollutants and requirements on monitoring of this set of materials, including antimony. In the US, companies are required to provide warning to California citizens about significant exposures to Antimony trioxide released in the environment, following the California proposition 65 or Safe Drinking Water and Toxic Enforcement Act of 1986 [International Antimony Association,NA].
Table 13 lists the countries for which the economic value of exports of Antimony represents more than 0.1 % in the total value of their exports. For all other exporting countries, this share is below 0.1 %.
| Country | Export value (USD) | Share in total exports |
|---|---|---|
Novel uses of antimony in catalysis
The high electrocatalytic activities of SnO2 doped with antimony (Sb), for two-electron oxygen reduction reaction (2e–-ORR) and one-electron water oxidation reaction (1e–-WOR) with extremely low affinity for H2O2 are promoted for use in H2O2 synthesis, low- temperature oxidative organic transformations, and water purification through the decomposition of organic pollutants . Sb is also used in selective catalytic reduction of NO with NH3 over V-Sb/Ti catalysts. The V-Sb/Ti catalyst showed excellent activity in the range 200–300 °C (compared with V/Ti), with an optimum achieved for 2 wt.% Sb [Kwon et al. ,2019].
Thin-film Sb Chalcogenide solar cells
Antimony is an ideal dopant due to its ionic radius similar to tin, so ATO, the antimony-doped tin(IV) oxide films, exhibit the lowest resistivity value of 1.23 x 10-2 ?cm and the highest transmittance of 73 % [Ponja S.D.,2018] [García-Vazquez, G.,2022] [García-Quiñonez, L.V.,2025].
ATOs can be applied in smart windows, lubricant oil, catalysts, sensors (humidity, gas), LIB & SIB battery additives, EMI shielding coatings, IR attenuation films & coatings.
TRL is in “Demonstration” for: lubricant oil, catalysts, sensors (humidity, gas), LIB & SIB battery additives, EMI shielding coatings, conductive coatings, conductive composites, IR attenuation films & coatings.
Application of Bi0.5Sb1.5Te3 (BST) alloys in thermoelectric membranes
Thin-film thermoelectrics structured as layers of Bi0.5Sb1.5Te3 (BST) alloys, have shown many attractive advantages, such as the micro cooling ability for solving heat dissipation issues in electronics and light mobile devices for harvesting human body heat. The high-quality Bi0.5Sb1.5Te3 (BST) epitaxial thin film on a sapphire substrate grown by spontaneous van der Waals epitaxy (vdWE) is exfoliated and transferred onto versatile materials, creating high-performance thermoelectric membranes. Unprecedented millimeter-size vdWE BST membranes are produced by etching a pseudomorphic Te monolayer on the surface of a sapphire substrate in a dilute HF solution. The intact exfoliation and direct transfer for vdWE BST membranes maintain the high-quality crystallinity, resulting in a remarkable zT value (∼0.9 at 300 K). These results represent the realisation of long-pursued but undemonstrated high-performance thin-film thermoelectric, paving the way for the design and fabrication of arbitrarily shaped thermoelectric devices [Fu et al.,2021].
A preparative approach to geometric effects in innovative solar cell types based on a nano cylindrical structure – SOLACYLIN (EU, 2015-2020)
The SOLACYN Project has developed an "extremely thin absorber" (ETA) solar cell materials system based on antimony sulphide as the light absorber. The deposition of individual layers by “Atomic layer deposition” ALD has enabled them to find by a systematic approach that the optimal thickness of antimony sulphide is 60 nm in a planar configuration. In addition, they have identified ZnSas as an interfacial layer which provides proper adhesion and anti-recombination barrier properties with an optimised thickness of 0.6 nm. They have transferred this interface engineering to coaxial nano cylindrical geometry and varied the length of the cylinders as well as the thickness of each layer. We have explored several materials as alternatives to classical semiconductors, MoS2, HfS2, SnO2, and V2O5 [Büttner et al.,2020].
Era Chair Of Emerging Next-Generation Photovoltaics 5gsolar – 5G SOLAR (EU, 2020-2026)
Different innovative photovoltaic solutions and products are needed to address the EU's significant environmental challenges in achieving and sustaining a green electricity market. The EU-funded 5GSOLAR project aims to further Europe’s sustainable development and clean energy goals and contribute to the European Research Area (ERA). To this end, it will converge research, development, and innovation as well as stakeholders, policymakers and society in the photovoltaics field. It will also create an European Research Area Chair team capable of implementing strategies and building a stakeholder network to help establish a renewable energy demo/briefing centre in Estonia and an EU joint graduate school in photovoltaics. The project will play a role in furthering Europe’s potential as a climate neutrality pioneer [ 5GSOLAR,2020].
Centre of Advanced Materials Research and Technology Transfer - CAMART (EU, 2017-2025)
The vision of CAMART is to establish ISSP as the most important centre of excellence for education, science, innovation, and technology transfer in the Baltic States. ISSP will also become the hub for a collaboration and technology transfer platform (called "RIX-STO"") for materials physics-based high technologies, including scientists, entrepreneurs, investors, and policymakers on both sides of the Baltic Sea. The commitment from the Swedish partners assures successful modernization of ISSP, including an overall refinement of the educational programs, strengthening of the research and development activities towards higher technology readiness levels, the establishment of an innovation system and Open Access Laboratory, as well as ISO 9001 certification. The partners will be active in an ambitious program for networking and outreach to make academia and high-tech industries in the region around Riga and Stockholm flourish concerning scientific results, economic growth, and increased competitiveness. The region will benefit from an injection of highly educated young people, closer collaboration between academia and industry, and offering state-of-the-art open access research infrastructure boosting innovation and economy [CAMART,2017].
ATMEN project (2017 -2022)
A recent discovery is that the scattering of the energetic imaging electrons can cause a silicon impurity to move through the graphene lattice has revealed a potential for atomically precise manipulation using the ?ngstr?m-sized electron probe. To develop this into a practical technique, improvements in the description of beam-induced displacements, advances in heteroatom implantation, and a concerted effort towards the automation of manipulations are required. This project tackles these in a multidisciplinary effort combining innovative computational techniques with pioneering experiments in an instrument where a low-energy ion implantation chamber is directly connected to an advanced electron microscope. To demonstrate the power of the method, an atomic memory will be prototyped with an unprecedented memory density and will create heteroatom quantum corrals optimized for their plasmonic properties. The capability for atom-scale engineering of covalent materials opens a new vista for nanotechnology, pushing back the boundaries of the possible and allowing a plethora of materials science questions to be studied at the ultimate level of control [ATMEN,2017].
Solution-processed antimony chalcogenides based thin film solar cells: A brief overview of recent developments (2022)
The search for an ideal absorber layer in thin-film solar cells seems to be a never-ending task. Apart from the solar absorber characteristics, antimony chalcogenide materials are gaining research interest predominantly due to their ribbon orientation and bandgap tunability in the entire solar spectrum. However, the challenges with open-circuit voltage deficit and low fill factor of solar cell devices remain unresolved. The reported highest power conversion efficiency with antimony chalcogenide absorber stays at 10.7%, where the absorber is made using a solution based hydrothermal synthesis method. In this mini-review, the latest developments related to solution-derived antimony chalcogenide absorber-based solar cells and the different strategies employed for improvements are presented in detail. With emphasis on the recent developments in the hydrothermal deposition of antimony chalcogenide absorber layer, the opportunities available to further enhance its properties through bandgap engineering and controlling the crystal orientation are discussed [Akshay et al.,2022].
The role of lone-pair electrons on electrocatalytic activity of copper antimony sulphide nanostructures (2022)
Crystal engineering is an elegant approach to the synthesis and design of active electrocatalysts for the large-scale production of hydrogen. Better realization of the crystal structure of the electrocatalyst assists the development of active catalysts. Different properties such as thermal properties, optical properties etc., appear to be largely affected by the presence of nonbonding lone-pair in the crystal structure. In this work, we have studied the influence of nonbonding electron pairs residing in the crystal structure on the electrocatalytic activity of the electrocatalysts. Herein, we report the synthesis of the three distinct crystal phases of copper antimony sulfide nanostructures named chalcostibite (CuSbS2), skinnerite (Cu3SbS3), and famatinite (Cu3SbS4), and studied their electrocatalytic activity in neutral medium. Chalcostibite and skinnerite phase show much better activity towards electrocatalytic hydrogen evolution reaction than the famatinite phase. This is explained due to the presence of antimony (Sb) lone pair electron in the former two cases compared to the latter [Hota et al. ,2022].
The separation behaviour of impurity in antimony during vacuum distillation (2022)
The models for depicting the variation tendencies of contents of Cu, Fe, Ni, Pb and Bi impurities in melt and distillate were established. The results proved that the mass transfer process was determined by the hybrid steps of mass transfer processes in the liquid boundary layer and at the liquid-gas interface for Pb and Bi impurities, whereas the rate-determining step was the mass transfer process at the liquid-gas interface for Cu, Fe and Ni impurities. The experimental results of Cu, Fe, Ni and Pb impurities in melt were coincident with the calculated results, while the experimental result was lower than the calculated result for the Bi impurity; the experimental result of Bi impurity in distillate was consistent with the calculated result, whereas there existed discrepancy for the Pb impurity; the marked increase of keff of Pb and Bi impurities during distillation were responsible for the difference between the experimental and calculated results in distillate. Additionally, the removal efficiencies of Cu, Fe and Ni impurities were higher than those of Pb and Bi impurities, the reason arose from that the vapor pressures of the latter were much closer to that of antimony than those of the former [Li et al.,2023].